Background:
The prognostic and predictive utility of measurable residual disease (MRD) assessments using bone marrow (BM) aspirates is well-established in the management of acute lymphoblastic leukemia (ALL). However, frequent BM MRD monitoring post-therapy is limited by the invasive, expensive, and at times impractical nature of numerous BM examinations. Limited retrospective reports have suggested that MRD analysis by next-generation sequencing (NGS) using peripheral blood (PB) may provide a viable alternative to MRD monitoring of the BM. We conducted a prospective, multi-institutional observational study of NGS-based MRD of the PB among adult ALL patients undergoing cellular therapies (hematopoietic cell transplantation [HCT] and chimeric antigen receptor T cells [CART]) in order to determine the correlation between PB and BM MRD and to explore the clinical utility of monitoring MRD in the PB.
Methods:
Patients >= 18 years-old with ALL were recruited from Stanford University and Kaiser Permanente, Northern California. The MRD analyses were conducted using Adaptive Biotechnologies ClonoSEQ NGS based platform that tracks tumor specific VDJ rearrangement in B and T cell malignancies. Assessment of MRD was obtained from the PB and BM prior to HCT/CART. Among HCT patients, PB MRD was obtained at one month, and then every 2-3 months for the first year following HCT; a paired BM MRD sample was obtained at the 3 month time-point with optional additional BM examinations. Among CART patients, paired PB and BM MRD were obtained at one month, and then every 2-3 months for the first year following CART. The correlation between log10 values of PB and BM MRD samples was evaluated using the Pearson correlation coefficient. Clinical relapse was defined as morphologic leukemia blasts in the marrow or extramedullary site, or administration of a new therapy for rising MRD.
Results:
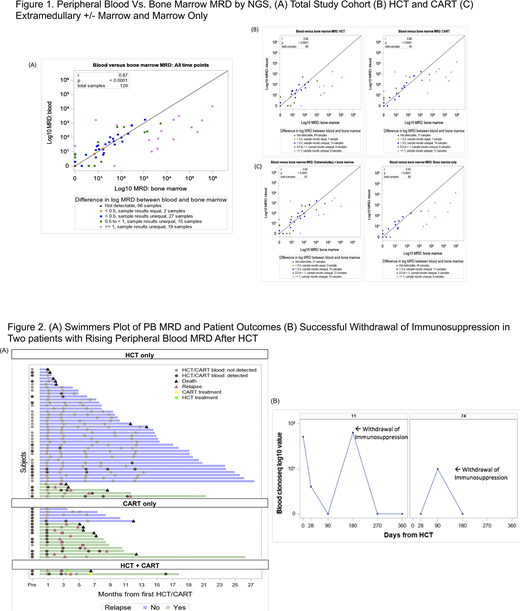
Sixty-nine patients scheduled to undergo cellular therapies were enrolled; 3 (4%) did not undergo planned therapy and were excluded and 4 (6%) lacked a detectable clonal leukemia sequence and were thus off study, resulting in a study population of 62 (42 BMT, 17 CART, 3 BMT and CART). The median age was 42 years (IQR 30-53), 36 (58%) were male, 54 (87%) had B-ALL,16 (26%) were BCR-ABL+, and 28 (46%) had extramedullary (EM) involvement. Across all patients, PB MRD was highly correlated with BM MRD (r=0.87; p<0.0001; Figure 1A). Of the 129 paired samples,15 (12%) had discordance with MRD identified in either the PB and not BM (N=7; 5%) or in the BM and not PB (N=8; 6%). Similarly, PB and BM MRD were highly correlated in the HCT (r=0.86; p<0.0001) and CART cohorts (r=0.86; p<0.001; Figure 1B), and among patients with EM involvement (r=0.83; p<0.0001) and marrow only disease (r=0.93; p<0.0001; Figure 1C). With median follow-up of 293 days (IQR: 180-512), 6 (13%) HCT and 13 (65%) CART patients experienced clinical relapse (Figure 2A). Among the 6 patients who relapsed following HCT, 80% had detectable MRD in the PB prior to HCT (1 was missing pre-HCT PB sample). Following HCT, all 6 patients developed detectable MRD, with median time from first MRD positivity to clinical relapse of 71 days (IQR 28-90). Among the 13 patients who relapsed following CART, 85% had detectable MRD in the PB a median of 60 days (50-139) prior to clinical relapse. Finally, serial monitoring of the PB following HCT averted clinical relapse through immunosuppression withdrawal in two patients with rising MRD post-HCT (Figure 2B), thereby directly impacting patient outcomes.
Conclusion:
This prospective observational study demonstrates a strong correlation between PB and BM NGS MRD results in ALL. These results show that non-invasive monitoring of PB-based MRD in ALL patients undergoing curative intent cellular therapies represents a viable alternative to serial BM examinations, providing clinically actionable information and the opportunity to intervene on impending clinical relapse.
Muffly:Adaptive: Research Funding; Amgen: Consultancy; Servier: Research Funding. Meyer:Orca Bio: Research Funding. Negrin:Amgen: Consultancy; Magenta Therapeutics: Consultancy, Current equity holder in publicly-traded company; BioEclipse Therapeutics: Current equity holder in private company; Biosource: Current equity holder in private company; UpToDate: Honoraria; KUUR Therapeutics: Consultancy. Rezvani:Pharmacyclics: Research Funding. Sidana:Janssen: Consultancy. Shiraz:ORCA BioSystems: Research Funding; Kite, a Gilead Company: Research Funding. Shizuru:Jasper Therapeutics, Inc: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees. Liedtke:Adaptive: Membership on an entity's Board of Directors or advisory committees; Caelum: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; GSK: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria. Miklos:Kite-Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding; Adaptive Biotech: Consultancy, Other: Travel support, Research Funding; Allogene Therapeutics Inc.: Research Funding; Novartis: Consultancy, Other: Travel support, Research Funding; Pharmacyclics: Consultancy, Other: Travel support, Patents & Royalties, Research Funding; Juno-Celgene-Bristol-Myers Squibb: Consultancy, Other: Travel support, Research Funding; Janssen: Consultancy, Other: Travel support; Miltenyi Biotec: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal